ACKNOWLEDGEMENT
Proteasome inhibitors potentiate anti-tumor effects of standard cytotoxic chemotherapy in childhood leukemia. Ixazomib is an oral proteasome inhibitor that has a shorter proteasome dissociation half-life than bortezomib, which leads to a higher tumor-to-blood ratio inhibition. We performed a phase 1 trial to determine the dose limiting toxicities (DLT), Recommended Phase 2 Dose (RP2D) of ixazomib in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (ALL) or lymphoma (LL).
Patients ≤ 21 years of age with relapsed or refractory ALL (with ≥ 5% detectable disease in the marrow) or LL were eligible. Ixazomib was combined with 28-day blocks of well-established, relapsed ALL backbone chemotherapy. Block 1 contained vincristine (1.5 mg/m 2/dose IV Days 1, 8, 15, 22), dexamethasone (5 mg/m 2/dose IV or oral twice daily Days 1-14), PEG-asparaginase (2500 IU/m 2/dose IV Days 2, 15) and daunorubicin (60 mg/m 2/dose IV Day 1) (VXLD). An optional Block 2 contained vincristine (1.5 mg/m 2/dose IV Day 1), dexamethasone (3 mg/m 2/dose oral twice daily Days 1-5), methotrexate (1000 mg/m 2/dose IV over 36 hours Day 8), PEG-asparaginase (2500 IU/m 2/dose IV Day 9), cyclophosphamide (440 mg/m 2/dose IV Days 15-19) and etoposide (100 mg/m 2/dose IV Days 15-19). An additional optional Maintenance Block contained vincristine (1.5 mg/m 2/dose IV Day 1), prednisone (20 mg/m 2/dose oral twice daily Days 1-5), mercaptopurine (75 mg/m 2/dose oral daily) and methotrexate (20 mg/m 2/dose oral weekly). Intrathecal therapy was given throughout with agents and dosing based on the patient's CNS disease status upon study entry. Ixazomib was given orally and tested at two dose levels (DL) (DL1: 1.6 mg/m 2/dose; DL2: 2 mg/m 2/dose) using a 3+3 design and was given during Block 1 on Days 1, 4, 8 and 11; during Block 2 on Days 1, 4, 8, 15 and 18 and during Maintenance on Days 1, 8 and 15. DLTs during Block 1 only were utilized to make DL escalation decisions and determine the RP2D. Patients without Down Syndrome (DS) entered Cohort A, used to determine the P2RD. Patients with DS were eligible for a 1 DL lagging descriptive-only Cohort B.
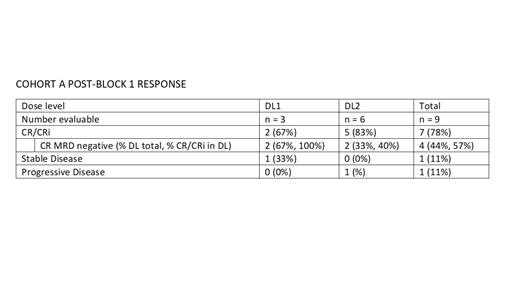
Ten patients were treated; nine in Cohort A (n = 3 at DL1 and n = 6 at DL2) and one in Cohort B (DL1). Median age (range) at study entry was 10.5 years (5 - 20 years). Nine patients had B-ALL and one had T-ALL. Most were heavily pretreated with a median (range) of 3.4 (1 - 7) previous remission attempts with six having had prior immunotherapy and three patients having had at least one prior hematopoietic stem cell transplant. After Block 1, two patients received Block 2 and one received Maintenance. In Block 1 of Cohort A, non-hematological Grade 3/4 adverse events (AE) possibly, probably or definitely related to ixazomib or the backbone chemotherapy were limited and included neutropenic fever (n = 7), increased AST (n = 3), increased ALT (n = 2) and hypokalemia (n = 2). Two septic events occurred (n = 1 in Cohort A (Grade 3) and n = 1 in Cohort B (Grade 4)). Block 2 and Maintenance AEs were not significantly different than those seen in Block 1. No deaths or DLTs occurred. Response assessment showed that at DL1 two of three patients had a Complete Remission (CR) both of whom were Minimal Residual Disease (MRD) negative (-) and at DL2 three of six patients had a CR, two of whom were MRD- and two patients had a CR with incomplete count recovery (CRi). The overall response rate (CR + CRi) was 78% (7 of 9 patients). With a minimum of 20 months of follow-up six of nine patients in Cohort A and the single patient in Cohort B are still alive. All three deceased patients died of disease progression.
Ixazomib can be combined with standard chemotherapy with an acceptable safety profile and an encouraging early efficacy signal in pediatric relapsed ALL including those with DS. The RP2D of Ixazomib combined with chemotherapy is 2 mg/m 2/dose, which is being used in the ongoing phase 2/expansion cohort of the study.
We acknowledge the TACL Consortium's scientific contribution to and participation in this study, including participating member institutions, investigators, research teams, and the TACL Operations Center. Takeda Pharmaceuticals Company provided the investigative drug and funding in support of this trial.
Disclosures
Schafer:Takeda: Research Funding. Rushing:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Rubnitz:Biomea, Inc: Consultancy. Horton:Takeda: Research Funding.